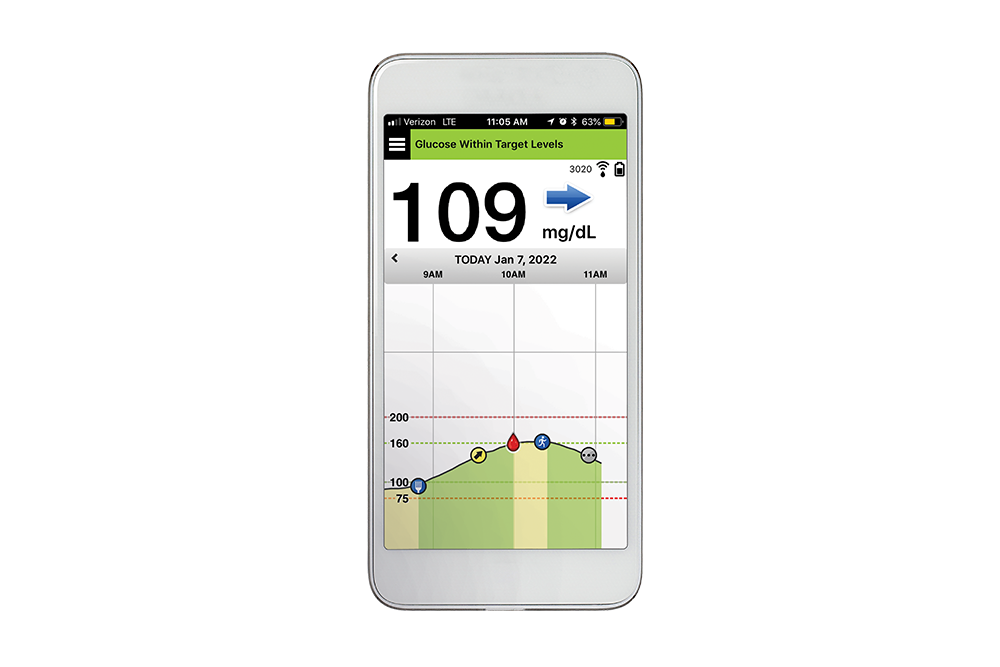
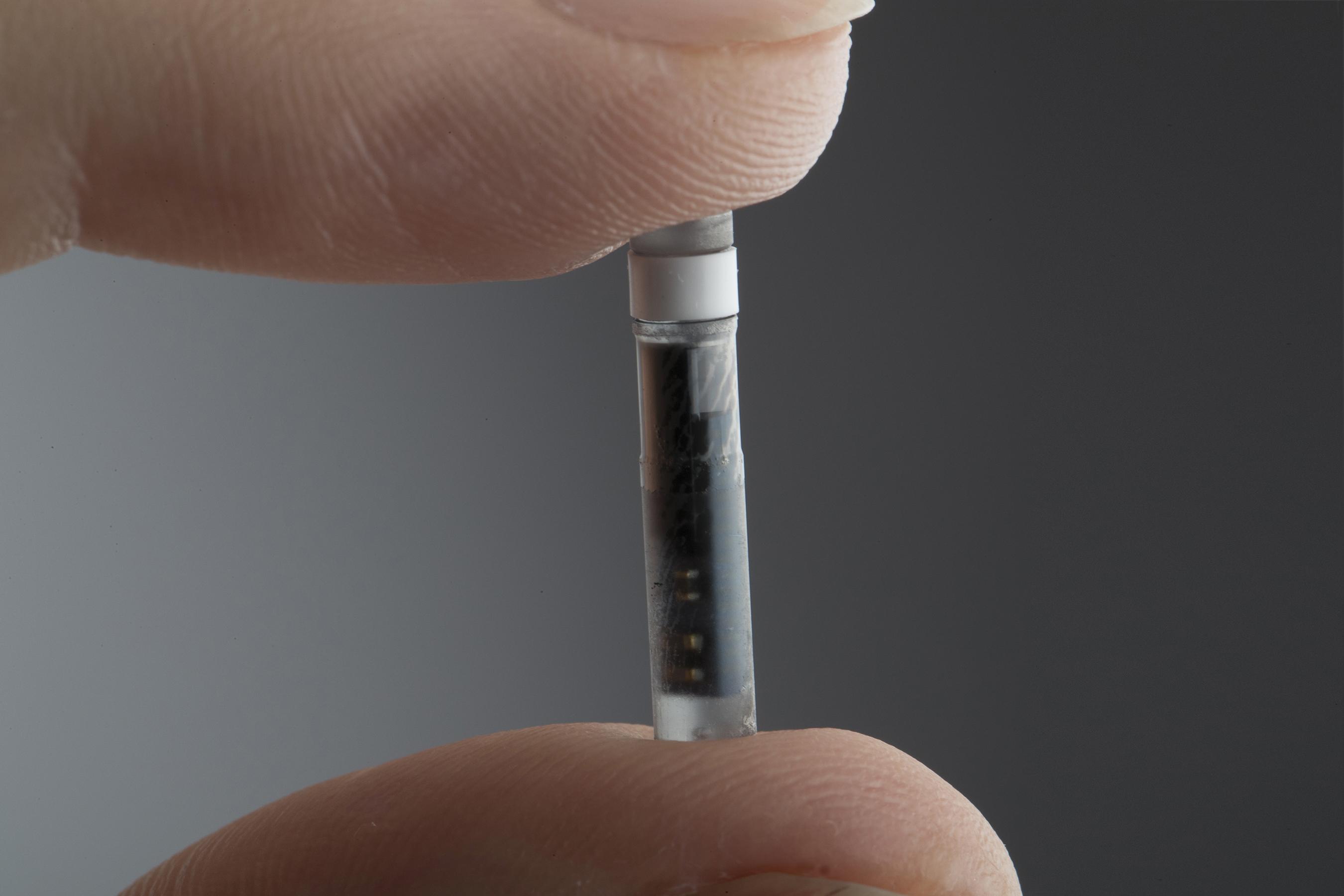
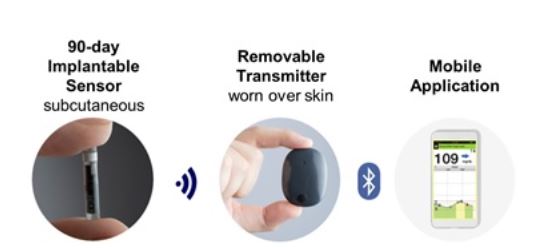
FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News

Implantable glucose sensor featuring IDT sensing technology awarded CE Mark - Medical Design and Outsourcing

Eversense sensor. CM, centimeter; DXA, dexamethasone acetate; PMMA,... | Download Scientific Diagram

Eversense E3 CGM Approved for Two Sensors per Year: Your “Happily Ever(sense) After” - Taking Control Of Your Diabetes®
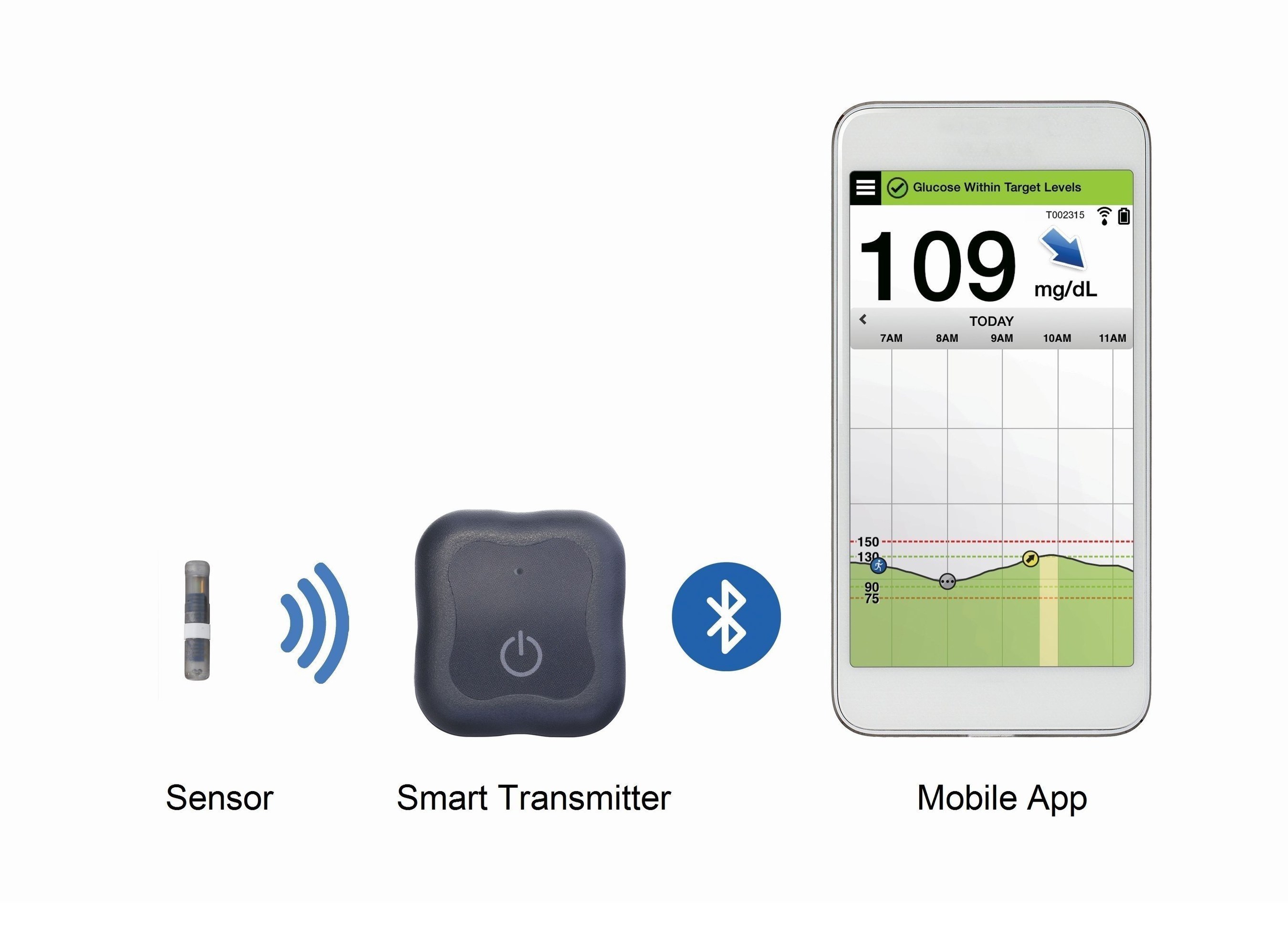
Roche Diabetes Care and Senseonics Announce Distribution Agreement for the Eversense® CGM System Introducing the First Implantable Long-term Glucose Sensor
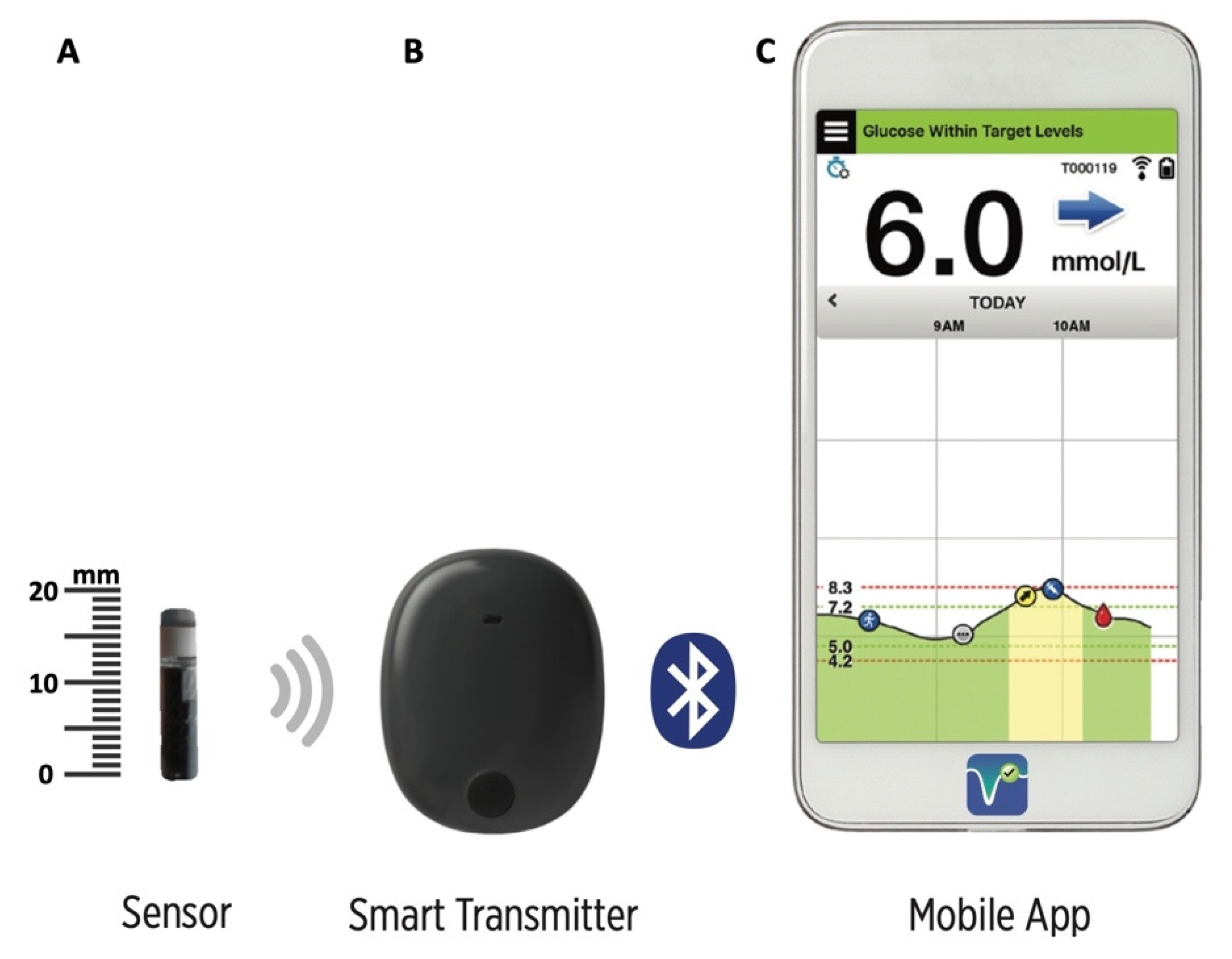
Animals | Free Full-Text | Clinical Use of a 180-Day Implantable Glucose Monitoring System in Dogs with Diabetes Mellitus: A Case Series